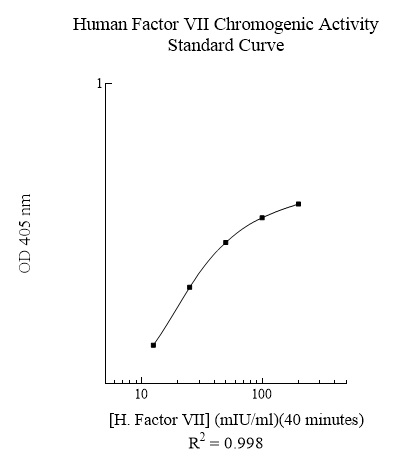
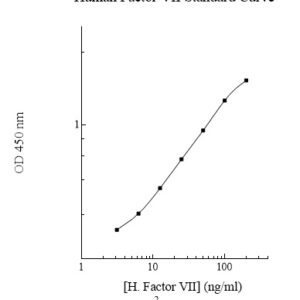
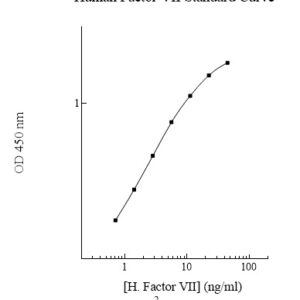
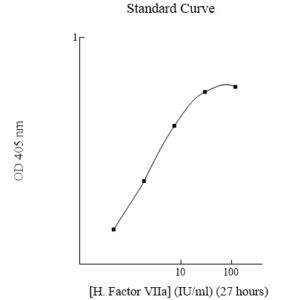
Publications (8 results)
Please use discretion when following external links; Assaypro cannot guarantee the validity and security of all citation links provided.* Koizume S et al. (2009) Hepatocyte Nuclear Factor-4-independent synthesis of coagulation factor VII in breast cancer cells and its inhibition by targeting selective histone acetyltransferases. Mol Cancer Res. 7(12): 1928-1936
* Fima UE et al. (2010) Long-Acting Coagulation Factors and Methods of Producing Same. Patent No: US 8476234 B2
* Krysiak R and Okopien B (2011) Haemostatic effects of simvastatin in subjects with impaired glucose tolerance. Intern Med J. 41(6):473-481
* Momot AP et al. (2014) Allowable values of different parameters of hemostatic system in physiological pregnancy. Kazakhstan Association of Medical Laboratory Diagnostics 4(11):49-59
* Dumont L et al. (2015) The bioequivalence of frozen plasma prepared from whole blood held overnight at room temperature compared to fresh-frozen plasma prepared within eight hours of collection. Transfusion. 55(3):476-84.
* Momot A at al. (2015) Dynamics of indicators of the hemostasis system in women during pregnancy and after childbirth. Laboratory Service. 2: 3-11.
* Momot AP et al. (2016) The Dynamics of the Hemostatic Parameters in Physiological Pregnancy and After Delivery. J. Hematol Blood Transfus. Discord. 3:005.
* Bhattacharjee J et al. (2017) Autologous NeoHep derived from chronic HBV patient's blood monocytes by upregulation of cMET signalling. Stem Cells Transl Med. 6(1):174-186.